Problem Description Pro: 5W2H Investigation Tool for FDA & EMA Compliance
Reliable shipping
Flexible returns
Are you one investigation away from an FDA warning letter? In the pharmaceutical compliance and food safety industries, 70% of CAPA investigations fail due to poor problem definition—leading to recalls, fines, and shutdowns. You're not alone if your team struggles with vague reports, missing root causes, and endless rework. But what if defining the problem correctly solved 50% of it—as Lean Six Sigma and regulatory experts confirm?
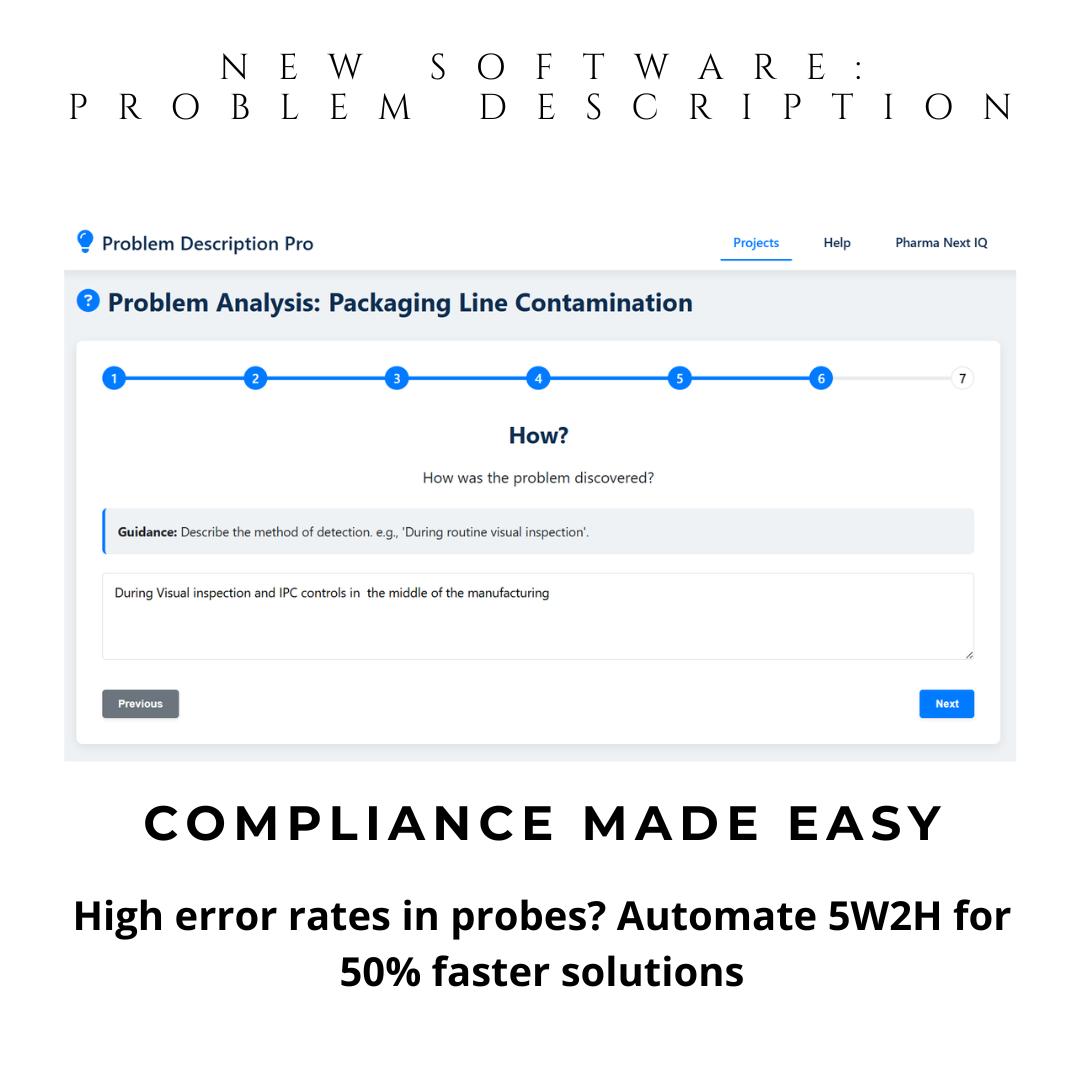
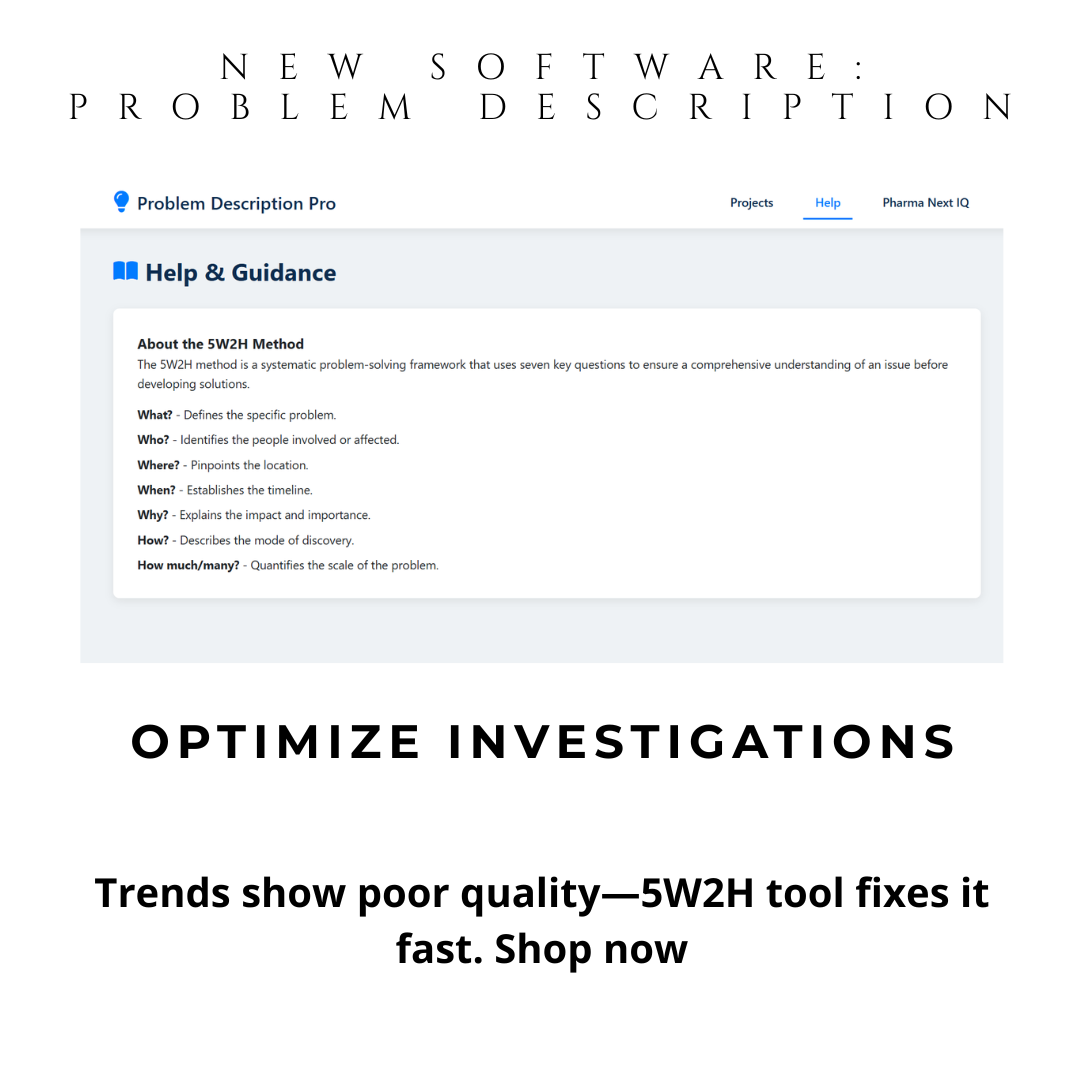
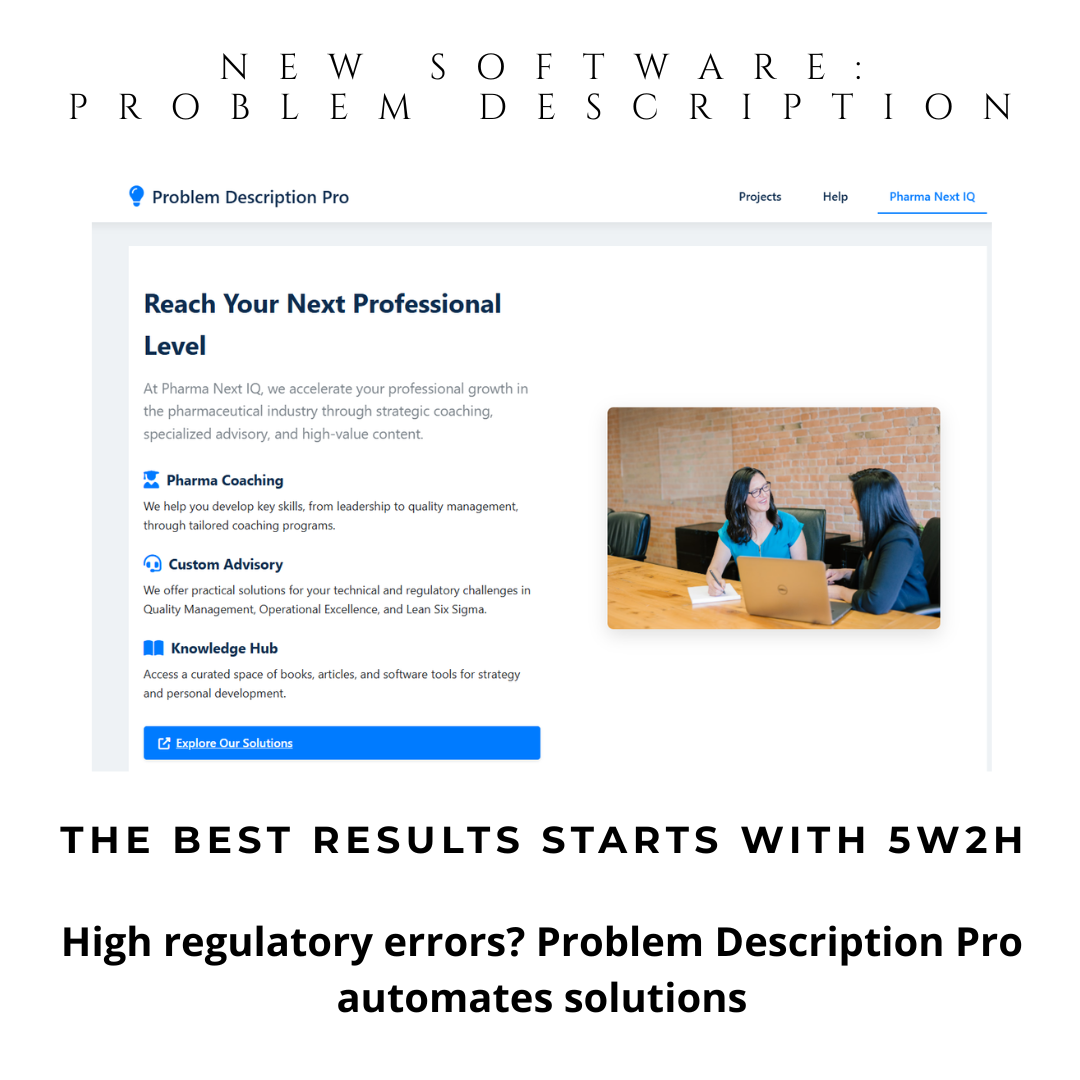
Problem Description Pro is your 5W2H investigation tool—a powerful digital CAPA software that automates problem definition using the proven 5W2H methodology (What, Who, Where, When, Why, How, How Much). No installation. No training. Just open on Windows or Mac and generate audit-ready problem statements in minutes.
Key Features
-
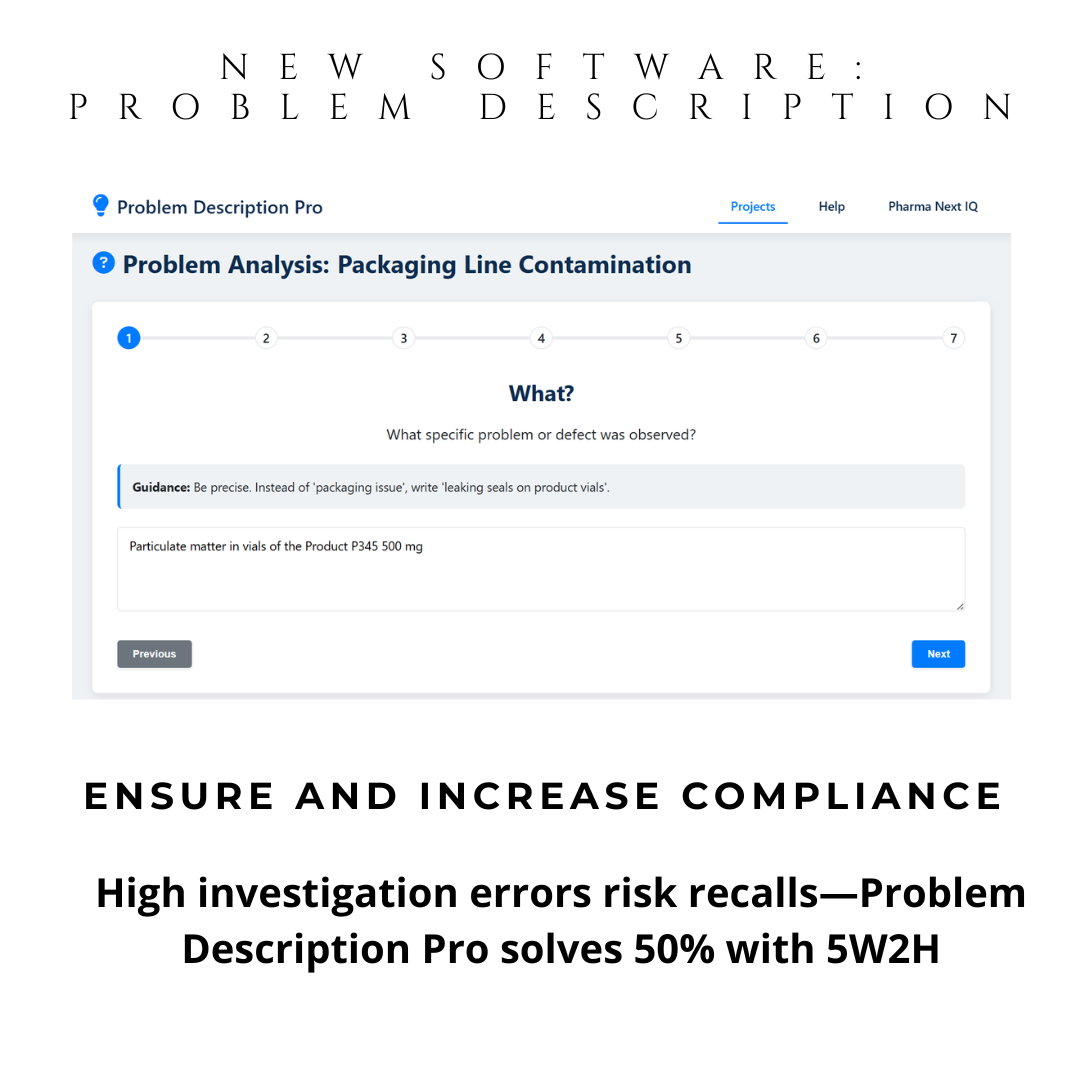
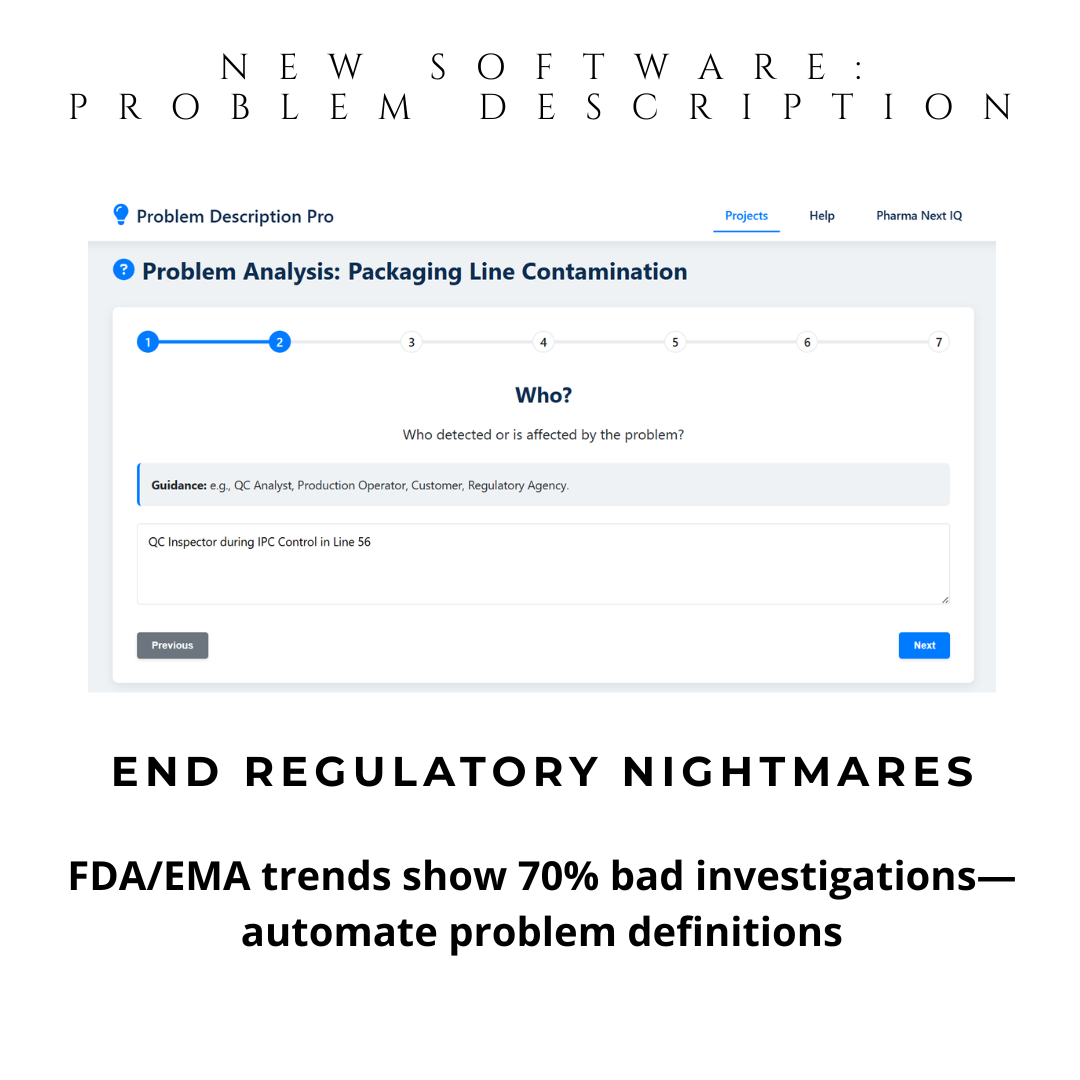
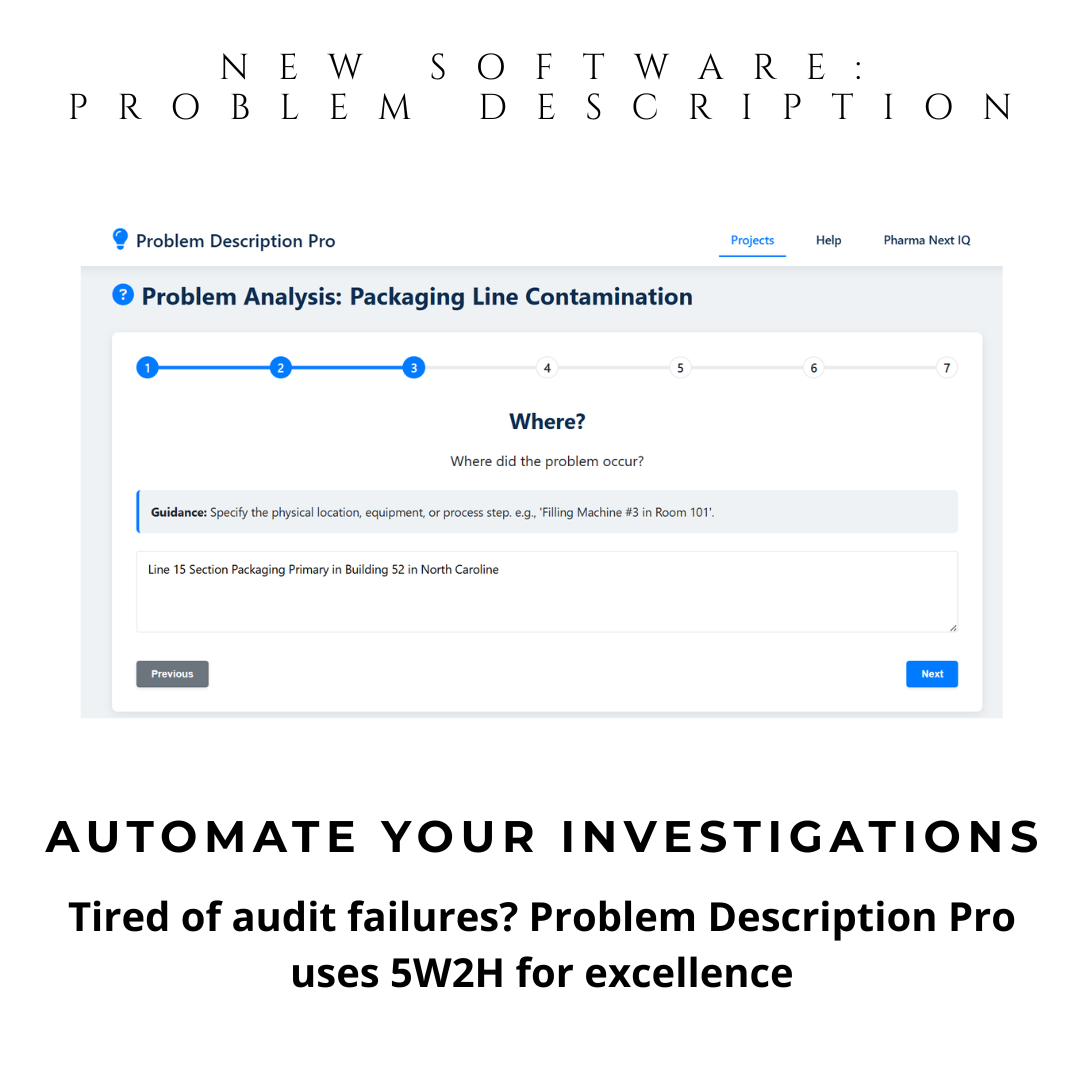
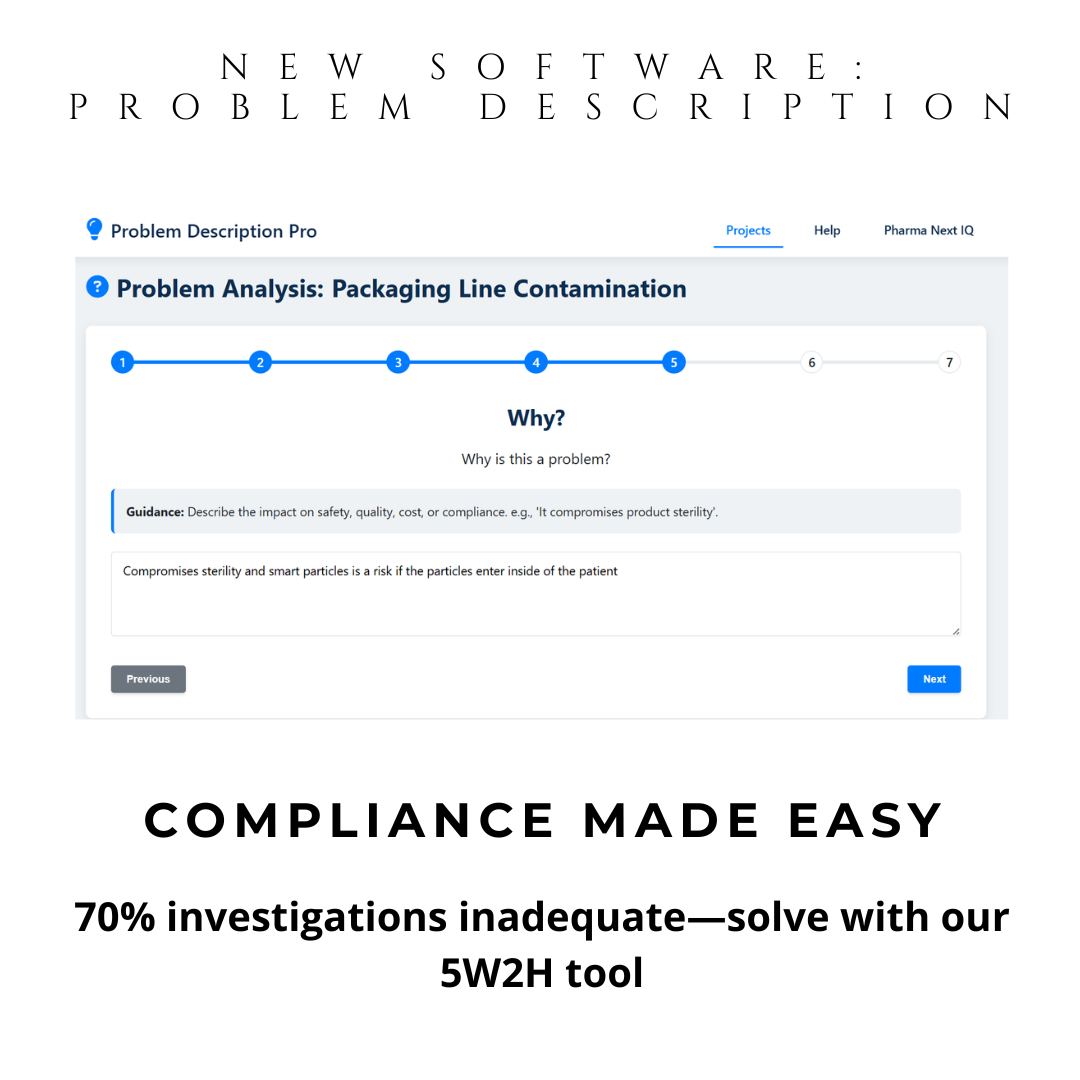
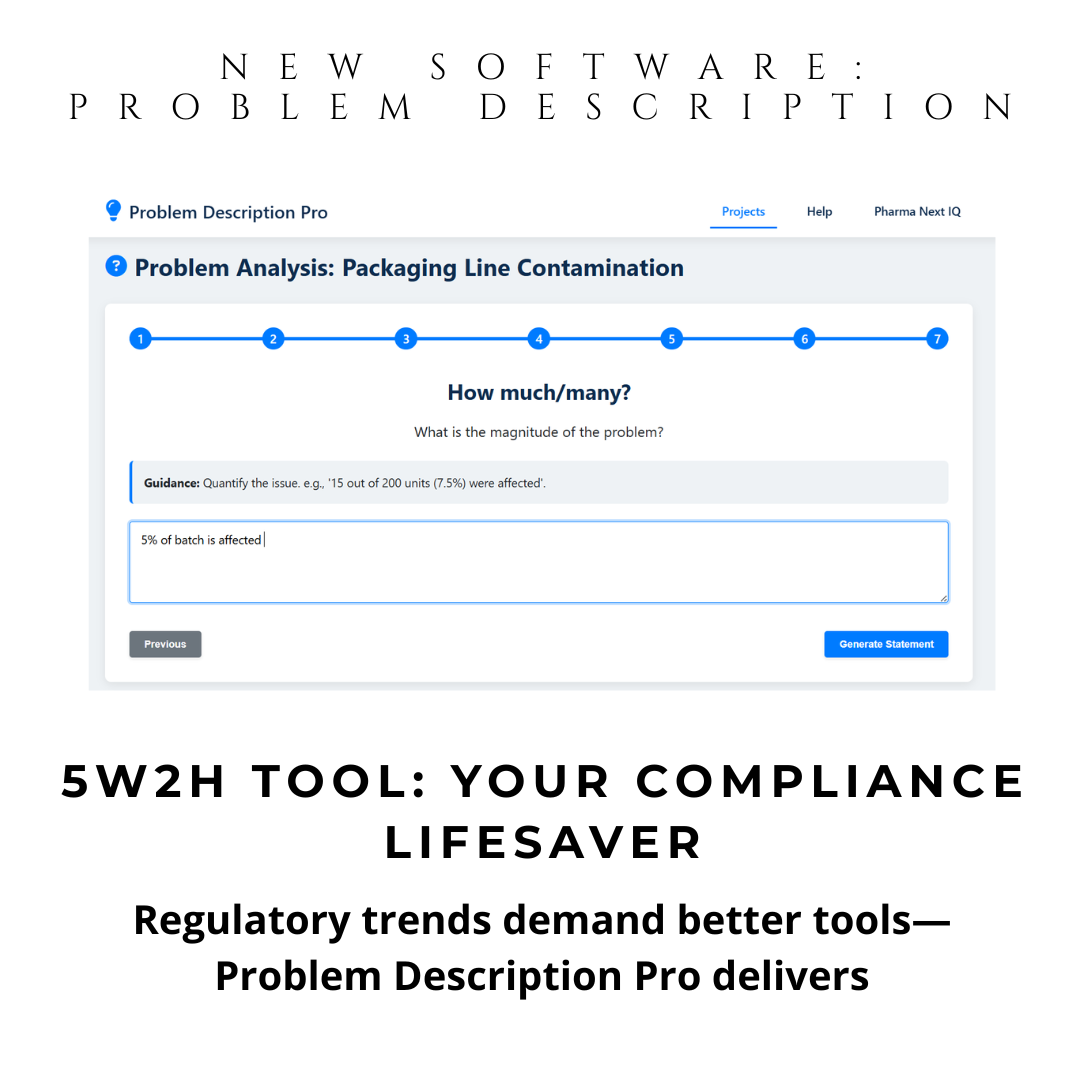
Instant 5W2H Automation: Guided questions generate structured problem descriptions. Ideal for preparation of your brainstorming sessions and discussion with SME investigators.
-
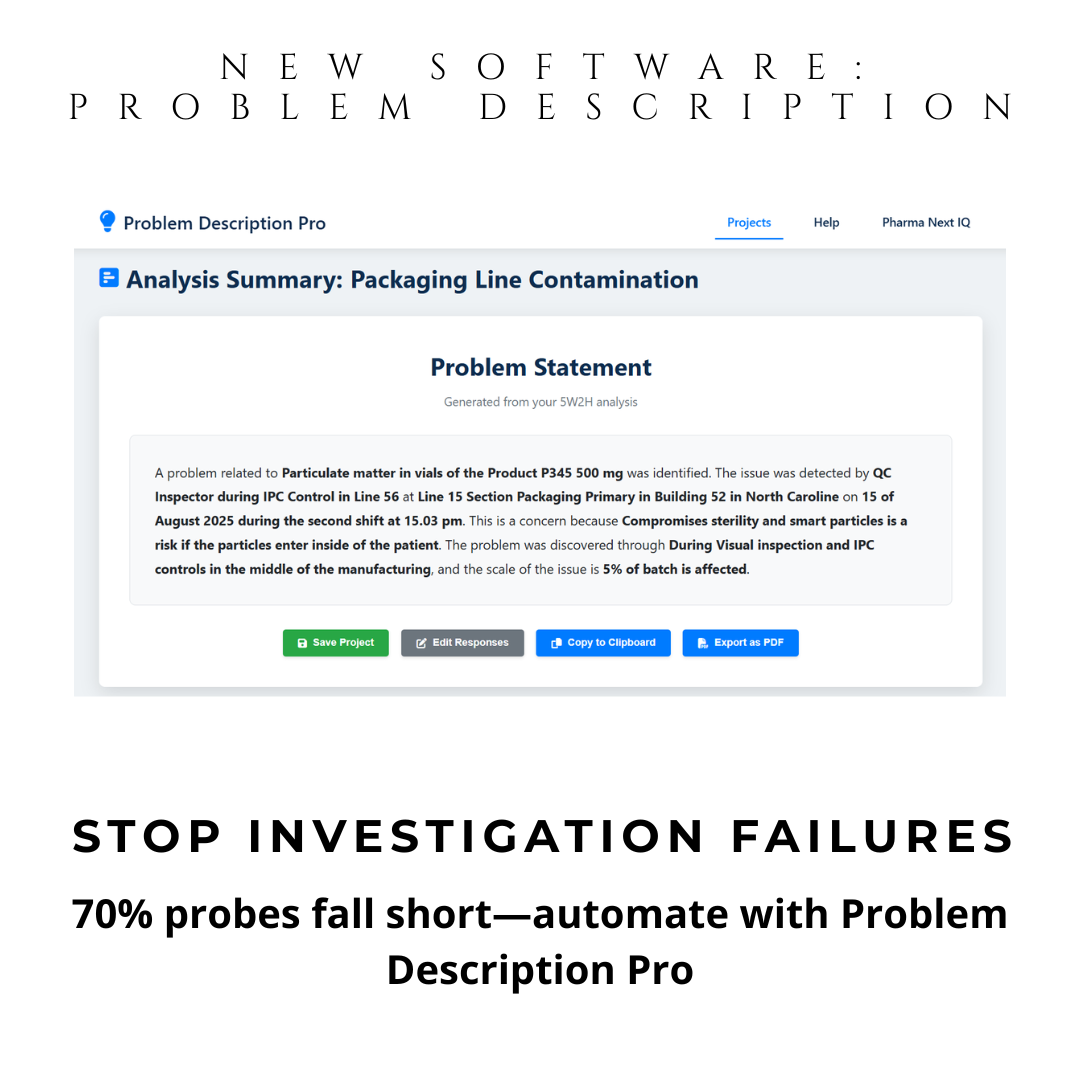
Auto-Summary Generation: One-click creation of CAPA-ready investigation summaries—export to PDF, Word, or clipboard.
- No Installation Required: HTML-based instant download tool—works offline, no IT approval needed.
Why This Tool Changes Everything
Imagine walking into your next audit with bulletproof problem descriptions that reduce investigation cycle time by 45% and eliminate 60% of rework. Lisa, a Quality Manager at a CDMO, used to dread FDA inspections. After switching to Problem Description Pro, for her brainstorming discussion to fix deviations.
“This tool turned our chaos into compliance confidence.” – Lisa, QP, EU Pharma
Why Buy Problem Description Pro?
Because regulatory scrutiny is rising—and poor investigation quality is the #1 cause of warning letters. Manual templates fail. Spreadsheets get lost. Generic apps lack structure.
Problem Description Pro generates the urgent need for precision by solving:
-
Vague “What happened?” statements
-
Missing “How much?” quantification
-
Non-auditable investigation records
-
Delayed root cause analysis
With automatic 5W2H guidance, you define problems precisely—the first and most critical step in any CAPA. This isn’t just a tool—it’s your compliance shield.
Trusted by 1,000+ users in GMP facilities worldwide. Developed by Pharma Next IQ—leaders in quality management software for regulated industries.
Limited-Time Launch Offer
Instant Digital Download Includes:
-
Problem Description Pro (Windows/Mac)
-
Printable 5W2H
- 100% Satisfaction Guarantee
Add to Cart Now and Secure Your Compliance Future